We seek to understand the mechanistic bases of zombie fly behaviors from the level of molecules to neural circuits.
Zombie flies are Drosophila melanogaster infected with Entomophthora muscae. These flies get their name because they act like zombies right before they die, performing bizarre behaviors that position them ideally for E. muscae dispersal:
1) Summit (climb to a high elevation)
2) Extend their proboscis
3) Raise their wings
Using a multidisciplinary experimental approach, we now understand key features of the pathway underlying summiting behavior.
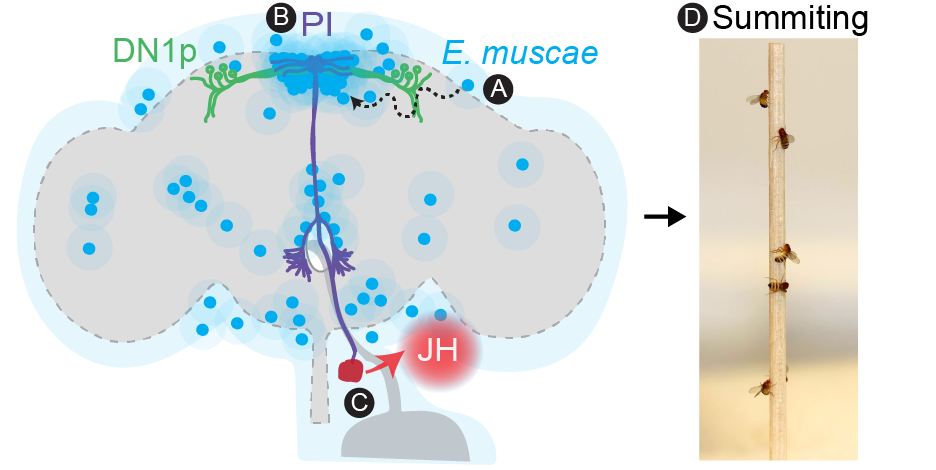
Proposed mechanism for summit behavior in zombie flies.
E. muscae cells invade brain in stereotyped pattern (A) then secrete neuromodulatory compounds that altering circadian (DN1p) and neurosecretory neuron (PI) activity (B). These activity changes lead to release of juvenile hormone (JH) (C) to ultimately drive summiting (D).
Some of the questions we’re most excited to next address include:
What effectors does E. muscae use to trigger summit disease?
Our data suggest that hemolymph-borne factor(s) in summiting flies can drive increased locomotion. What are these factors? Who makes them and by what mechanism? Where do they act? How is their production regulated?
Is invading the host brain necessary to drive zombie behaviors?
E. muscae eerily infiltrates the host brain well before the onset of zombie behaviors. But is this a requirement in order for those behaviors to happen?
How is the host clock altered by E. muscae infection?
Zombie flies appear to become arrhythmic on their last day of life. How does this occur? We hypothesize that understanding this phenomenon can help us better understand circadian dysregulation in animals more broadly.
We use a combination of behavior, genetics, imaging, molecular biological and biochemical approaches to address these questions.
There are a million and one open questions in the field of zombie fly and E. muscae biology. We’d love to know it all, but we can’t do it alone. We are actively seeking graduate students to join the effort! We are also happy to discuss possible collaborative opportunities in this new and exciting system.